Hardness test failure during tablet compression in SCADA system can impact product quality. Learn 10 causes, solutions, and CAPA with real-time corrective actions.

In pharmaceutical manufacturing, tablet hardness is one of the most critical quality attributes monitored during in-process checks. Hardness ensures that tablets remain intact during coating, packaging, transportation, and patient handling.
When a hardness test fails during tablet compression in a SCADA-controlled system, it indicates a process deviation. Unlike friability or dissolution failures, hardness issues are usually linked to compression force, tooling, lubrication, blend properties, or machine calibration.
This article explains the major reasons for hardness test failure at the compression stage, immediate corrective actions during machine running, required investigation, and CAPA (Corrective and Preventive Actions).
Reasons for Hardness Test Failure During Tablet Compression
Below are the 10 most common causes of hardness test failure observed specifically at the tablet compression stage:

1. Insufficient Main Compression Force
Reason: If main compression pressure is too low, inter-particle bonding is weak, leading to soft tablets.
SCADA Indication: Compression force trending below set target, tablets breaking easily.
2. Excessive Compression Force
Reason: Very high pressure causes capping, lamination, or internal stress, making tablets brittle.
SCADA Indication: Frequent capping alarms, abnormal thickness variations.
3. Uneven Granule Distribution in Hopper
Reason: Segregation in hopper leads to variable density tablets (hard-soft variations).
SCADA Indication: Wide fluctuations in tablet hardness within the same sample set.
4. Punch & Die Wear or Damage
Reason: Worn punches/dies fail to compact powder uniformly.
SCADA Indication: Higher weight/thickness variation alarms.
5. Excess Lubrication
Reason: Over-mixing with lubricants like magnesium stearate reduces particle bonding.
SCADA Indication: Tablets appear smooth, glossy but weak in strength.
6. Insufficient Lubrication
Reason: Low lubrication increases friction, leading to sticking/picking → inconsistent hardness.
SCADA Indication: Machine load variations, sticking alarms triggered.
7. Improper Tablet Thickness Setting
Reason: Too thick = low hardness; too thin = brittle tablets.
SCADA Indication: Thickness deviation alarms.
8. Moisture Variation in Blend
Reason: Over-dried or under-dried granules reduce compressibility.
SCADA Indication: Sudden changes in tablet hardness with shift in RH/temperature trends.
9. Poor Machine Calibration
Reason: Load cells and hardness testers not calibrated, giving false readings.
SCADA Indication: Discrepancies between SCADA data and offline lab test results.
10. Environmental Factors (RH / Temp.)
Reason: High humidity makes granules sticky, while low humidity causes friable tablets.
SCADA Indication: Environmental alarms linked with HVAC monitoring
Immediate Corrective Actions During Machine Running
When hardness test failure is detected mid-production, the following actions should be taken without delay:
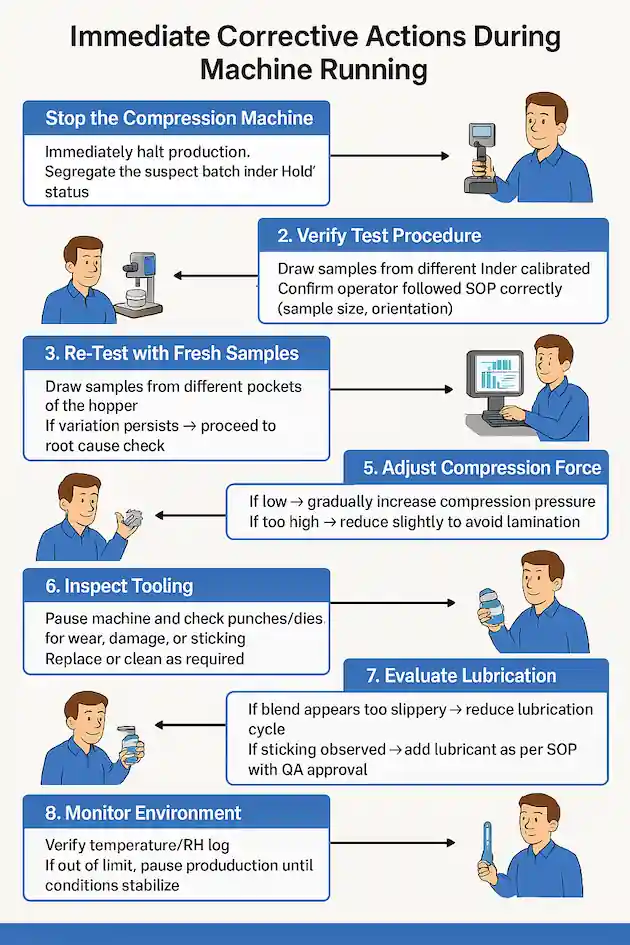
Step 1: Stop the Compression Machine
Immediately halt production.
Segregate the suspect batch under “Hold” status.
Step 2: Verify Test Procedure
Ensure friability/hardness tester is calibrated.
Confirm operator followed SOP correctly (sample size, orientation).
Step 3: Re-Test with Fresh Samples
Draw samples from different pockets of the hopper.
If variation persists → proceed to root cause check.
Step 4: SCADA Parameter Review
Check main compression force trend.
Check thickness & weight control limits.
Verify pre-compression vs. main compression pressure.
Step 5: Adjust Compression Force
If low → gradually increase compression pressure.
If too high → reduce slightly to avoid lamination.
Step 6: Inspect Tooling
Pause machine and check punches/dies for wear, damage, or sticking.
Replace or clean as required.
Step 7: Evaluate Lubrication
If blend appears too slippery → reduce lubrication cycle.
If sticking observed → add lubricant as per SOP with QA approval.
Step 8: Monitor Environment
Verify temperature/RH log.
If out of limit, pause production until conditions stabilize.
Required Actions if Test Failed During Machine Running
If failure is confirmed:
Quarantine the affected lot – Mark all compressed tablets as “Under Investigation”.
Raise Deviation Report – Document in BMR and SCADA deviation log.
Initiate OOS (Out of Specification) investigation – QA to lead.
Check Machine Calibration – Verify SCADA sensors, load cells, and hardness tester calibration.
Perform Root Cause Analysis (RCA) – Use tools like 5-Why or Fishbone Diagram.
Batch Disposition – Depending on findings, decide:
Re-compression (if blend is good and root cause corrected).
Rejection (if blend defective/unrecoverable).
CAPA (Corrective and Preventive Actions)
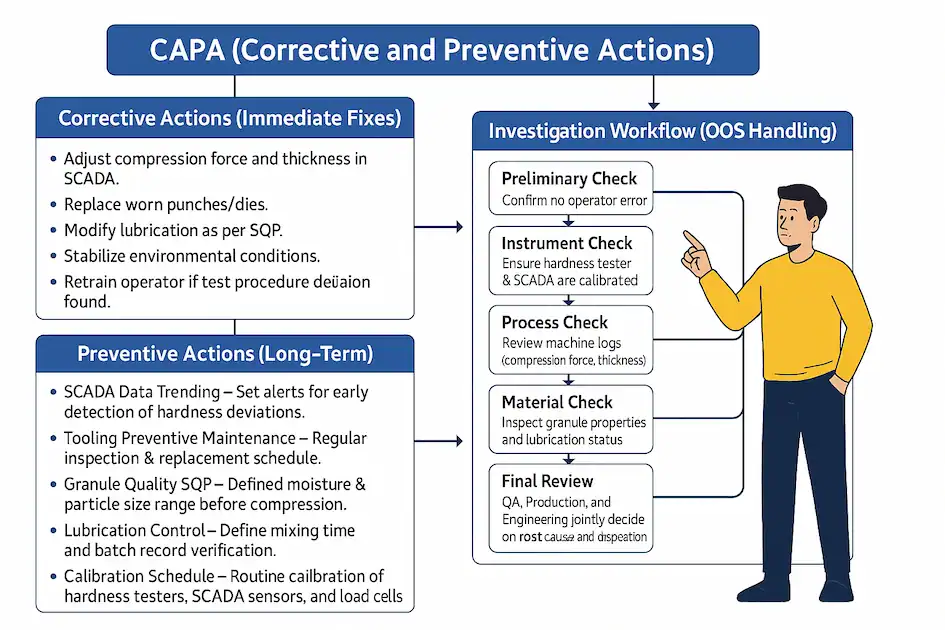
Corrective Actions (Immediate Fixes)
Adjust compression force and thickness in SCADA.
Replace worn punches/dies.
Modify lubrication as per SOP.
Stabilize environmental conditions.
Retrain operator if test procedure deviation found.
Preventive Actions (Long-Term)
SCADA Data Trending – Set alerts for early detection of hardness deviations.
Tooling Preventive Maintenance – Regular inspection & replacement schedule.
Granule Quality SOP – Defined moisture & particle size range before compression.
Lubrication Control – Define mixing time and batch record verification.
Calibration Schedule – Routine calibration of hardness testers, SCADA sensors, and load cells.
Operator Training – Periodic refresher training on in-process checks.
Environmental Monitoring – Continuous monitoring with auto-shutdown if out of limit.
Investigation Workflow (OOS Handling)
Preliminary Check – Confirm no operator error.
Instrument Check – Ensure hardness tester & SCADA are calibrated.
Process Check – Review machine logs (compression force, thickness).
Material Check – Inspect granule properties and lubrication status.
Final Review – QA, Production, and Engineering jointly decide on root cause and disposition.
निष्कर्ष
Hardness test failure during tablet compression in SCADA systems is a serious manufacturing deviation but can be managed effectively if detected early.
By: Monitoring SCADA parameters in real-time, Taking immediate corrective actions during running, Conducting systematic OOS investigation, and Implementing CAPA with preventive measures, manufacturers can ensure consistent, strong, and compliant tablets while avoiding costly rejections and regulatory risks.
What causes hardness test failure during tablet compression?
Answer: Common causes include low or high compression force, poor granule quality, excess lubrication, worn punches/dies, or improper machine calibration.
How does SCADA help in monitoring tablet hardness?
SCADA tracks compression force, weight, and thickness in real time. By trending these parameters, operators can detect early signs of hardness deviation and take corrective action.
What immediate action should be taken if hardness test fails during compression?
Stop the machine, quarantine the batch, recheck test accuracy, review SCADA data, adjust compression force, inspect tooling, and inform QA for investigation.
Can hardness failure be corrected without rejecting the batch?
Yes, if the root cause is identified early (e.g., low compression force or worn tooling), corrective actions like force adjustment or punch replacement may restore compliance. QA must approve any reprocessing.
What CAPA is required after hardness test failure in pharma manufacturing?
CAPA includes corrective actions like machine setting adjustments and preventive actions such as tooling maintenance, SCADA data trending, calibration schedules, and operator training.
Along with pharma manufacturing insights, I also write about personal finance. You can explore my latest guides on Best Mutual Funds and Top Credit Cards

