
Understand the 5 major reasons for friability test failure during tablet compression stage and explore detailed, research-backed solutions to fix friability issues in pharmaceutical tablet manufacturing.
In the pharmaceutical industry, tablet compression is one of the most critical stages in solid dosage manufacturing. Even when the granulation and formulation are perfect, problems such as friability failure can still occur during the compression process.
Friability is a measure of how easily a tablet can crumble, chip, or break under mechanical stress during handling, coating, packaging, and transportation. According to pharmacopeial standards (USP, IP, Ph. Eur.), tablets must not lose more than 1% of their weight during the friability test. If tablets fail this test, it not only leads to batch rejection but also raises regulatory and quality concerns.This blog provides a deep dive into the exact reasons for friability failure during the tablet compression stage and offers practical, research-based solutions for each problem.What is Friability Test and Why It Matters?
The friability test is performed using a Roche friabilator where tablets are rotated at 25 rpm for 4 minutes (100 rotations). After the test, tablets are weighed to calculate weight loss.
Acceptable Limit: ≤ 1% weight loss
Reason for Failure: Poor mechanical strength of tablets.
Impact: Chipping, breaking during handling, poor market acceptability, regulatory non-compliance Reasons for Friability Test Failure During Tablet Compression.
Below are 5 compression-related reasons why friability test failure during tablet compression, even if formulation and granulation are correct.
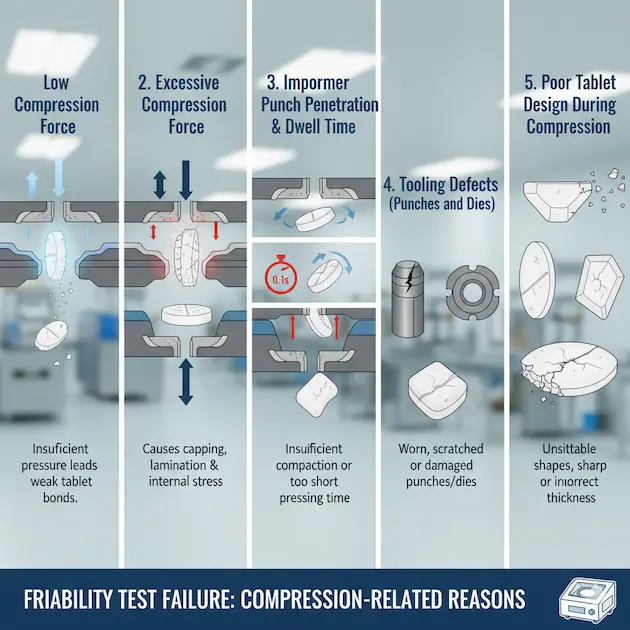
1. Low Compression Force
If the applied compression force is too low, particles do not bond strongly, resulting in weak tablets.
Tablets remain soft, fragile, and easily chip during friability testing.
Example: A tablet compressed at 5 kN instead of the required 10–12 kN will not have sufficient hardness and will fail friability.
2. Excessive Compression Force
Contrary to the above, very high compression force can also cause friability problems.
Excess force creates internal stress, leading to lamination or capping.
Although tablets may appear hard externally, micro-cracks make them fail in friability tests.
Example: A tablet compressed at 25 kN develops invisible stress lines; during friabilator rotation, these stress lines cause breakage.
3. Improper Punch Penetration & Dwell Time
At high machine speeds, the dwell time (contact time between punch and tablet bed) decreases.
Reduced dwell time results in poor particle bonding.Incorrect punch penetration depth also leads to uneven compaction and friability issues.
Example: On a high-speed rotary press, if dwell time is <20 ms, particles don’t get enough time to bond properly.
4. Tooling Defects (Punches and Dies)
Worn-out, scratched, or damaged punches and dies directly affect tablet quality. Sharp edges break easily, especially in flat tablets.Uneven die walls cause stress concentration, leading to friability failures.
Example: A punch with worn cup edge produces thin, fragile tablets that chip during handling.
5. Poor Tablet Design During Compression
Tablets with sharp edges, very thin design, or flat surfaces are more prone to friability.Convex or round tablets withstand mechanical stress better than flat ones.
Example: A flat 8 mm tablet with sharp edges will fail more easily compared to a biconvex 8 mm tablet of the same formulation.Solutions for Friability Failure During Tablet Compression
Let us now look at practical solutions for each of the above problems of friability test failure during tablet compression
1. Optimize Compression Force Increase
compression pressure to ensure adequate hardness.Avoid excessive force to prevent lamination and capping.Conduct hardness vs. friability trials to establish the optimal force range.
2. Adjust Pre-Compression and Main Compression
Balance Pre-compression removes entrapped air before final compaction.Maintaining the right balance between pre-compression and main compression ensures strong inter-particle bonding.
Example: Increase pre-compression force slightly to remove air → reduces friability failures.
3. Improve Punch Penetration & Dwell Time
Slow down the machine speed to allow better bonding.Use dwell-time enhancing cams in high-speed machines.Set proper upper/lower punch penetration depth.Practical Tip: Increasing dwell time by just 10–15 ms can significantly reduce friability issues.
4. Maintain & Replace Tooling
Regularly Inspect punches and dies daily for wear and tear. Replace worn-out tooling immediately. Use polished, high-quality punches to reduce edge stress. Avoid re-using damaged tooling, as it compromises batch quality.
5. Modify Tablet Shape at Compression Stage
Prefer round or biconvex tablets instead of thin, flat tablets. If regulatory constraints allow, adjust tablet dimensions for durability.
If friability test fails during tablet compression in a SCADA-controlled system, it means the tablets are not mechanically strong enough and may break/chip during handling, coating, packing, or transportation. In a regulated pharma environment (GMP), here’s the structured approach you should follow:
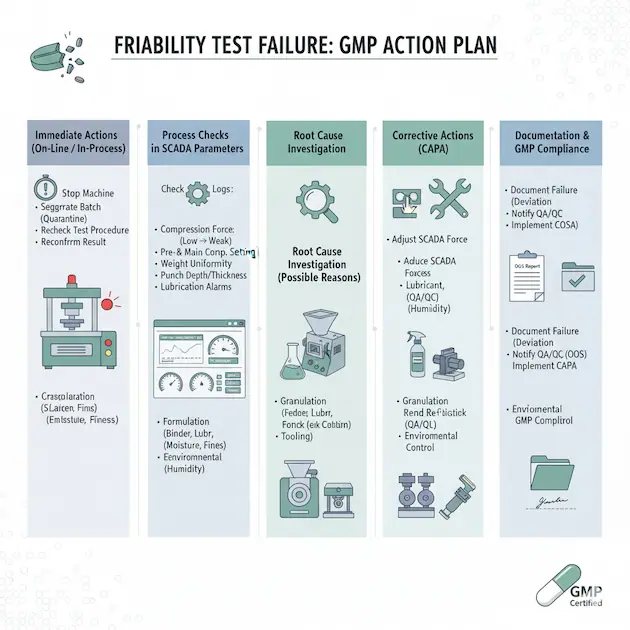
1. Immediate Actions (On-Line / In-Process)
Stop the compression machine – Do not continue until investigation is started.Segregate the affected batch – Keep the suspect tablets in quarantine status.Recheck test procedure – Ensure the friability test was performed correctly (RPM, time, sample weight, pre-dedusting). Sometimes operator error causes false failure.Reconfirm result – Retest with a fresh sample from different pockets of the batch.
2. Process Checks in SCADA Parameters
Friability is linked with tablet hardness, weight, thickness, and formulation factors. Check in SCADA logs for:
- Compression force – Too low → weak tablets.
- Pre-compression & main compression settings – Adjust gradually to increase hardness without causing capping/lamination.
- Tablet weight uniformity – Weight variation can reduce mechanical strength.
- Punch penetration depth / thickness – Ensure proper setting.
- Lubrication alarms – Excess lubricant (e.g., magnesium stearate) reduces bonding, causing friability issues.
3. Root Cause Investigation
(Possible Reasons)Formulation-related: Poor binder, excessive lubricant, hygroscopic excipients.Granulation-related: Low moisture, improper drying, large fines or oversized granules.Compression-related: Low compression force, worn-out punches/dies, misalignment.Environmental: High humidity leading to weak tablets.
4. Corrective Actions (CAPA)
Adjust compression force within the SCADA system.Recheck lubrication level – Reduce excess glidant/lubricant.Blend re-lubrication if possible (with QA/QC approval).Granulation rework – If tablets consistently fail, the batch may require re-granulation or blending with additional binder (as per SOP).Replace or polish tooling if punches/dies are worn.Environmental control – Ensure RH and temperature are within limits.
5. Documentation & GMP Compliance
Document failure in BMR/SCADA log with deviation report.Notify QA/QC for investigation and decision (reprocessing / rejection).Initiate OOS (Out of Specification) procedure if applicable.Implement CAPA before restarting batch compression.
Research and Industry Best Practices
ICH Q8 guidelines recommend continuous monitoring of critical process parameters (CPPs) like compression force. Research shows that increasing dwell time by 15 ms can reduce friability by 25–30%.
A 2023 study in International Journal of Pharmaceutics highlighted that tooling wear is a leading cause of friability failure in high-speed compression. USP and Ph. Eur. standards allow ≤ 1% friability weight loss, making machine adjustments critical for compliance.
निष्कर्ष
Friability test failure in tablet compression is a frequent challenge faced in pharmaceutical manufacturing. Unlike formulation-related problems, these failures are directly linked to machine settings, tooling conditions, and tablet design.
By: Optimizing compression force, Balancing pre- and main compression, Adjusting dwell time and punch penetration, Maintaining tooling quality, and Choosing durable tablet designs manufacturers can significantly reduce friability issues and ensure consistent tablet quality, regulatory compliance, and smooth production.
What is friability test in tablet manufacturing?
The friability test checks the ability of tablets to withstand mechanical stress like handling, coating, and packaging. If tablets lose more than 1% weight after testing in a friabilator, they fail.
Why do friability test failure during compression?
Tablets may fail due to low compression force, excessive compression force, short dwell time, tooling defects, or poor tablet design.
How can I reduce friability during tablet compression?
You can reduce friability by optimizing compression pressure, adjusting dwell time, balancing pre- and main compression, maintaining punch & die quality, and modifying tablet shape.
What is the acceptable limit for friability according to USP?
According to USP and IP standards, friability loss should not exceed 1% of tablet weight.
How does tablet design affect friability?
Flat or thin tablets with sharp edges are more prone to friability, while biconvex or round tablets are stronger and less likely to fail.
Apart from pharma, I also write about personal finance and smart money management. You can explore my latest guides on Mutual Funds and Best Credit Cards.”

